Dropper (EUP)
Droppers are simple yet essential tools designed for precise liquid measurements, especially in pharmaceutical and laboratory applications. Designed to transfer small amounts of liquid in a controlled manner, droppers are subject to strict quality control in terms of standardization and reproducibility within the fields they are used. This article details the structural properties, areas of use, European Pharmacopoeia compliance criteria, and quality control processes for droppers.
Structural Properties of Droppers
The structural properties of droppers are meticulously designed to allow liquid flow at a specific rate and in a controlled manner. Standard droppers are usually made of transparent glass material and have the following characteristics:
- Lower End Structure: The lower end of the dropper has a circular opening on a plane perpendicular to its axis. This design allows the drops to flow at a consistent rate and volume when held in a vertical position.
- Dimensions and Volume Calibration: The dimensions and aperture diameter of the dropper are designed to enable each drop to form at a steady rate, ensuring consistency in terms of weight and volume.
Meeting the structural requirements of standard droppers allows accurate dosage application, especially in pharmaceutical settings where precision is essential.
Droppers According to European Pharmacopoeia (EUP) Standards
The European Pharmacopoeia establishes standards for droppers, requiring each drop to meet specific weight and volume criteria through certain tests and regulations. According to 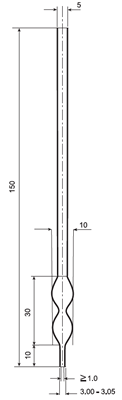 EUP standards, the following tests are conducted to determine the compliance of a dropper:
EUP standards, the following tests are conducted to determine the compliance of a dropper:
- Weight Standard: A total of 20 freely flowing drops of water (at 20 ± 1 °C) from the dropper should weigh 1000 ± 50 mg, meaning each drop should weigh approximately 50 mg. This parameter is crucial, especially in situations where precise pharmaceutical dosing is required.
- Flow Rate: The dropper should be held in a vertical position, and each drop should fall at a rate of one drop per second. This rate is essential to maintain a consistent volume of liquid release over a set period.
- Repeatability and Precision Test: Three measurements should be performed on the same dropper, with a deviation of no more than 5% from the average. This test ensures that the dropper consistently dispenses the same volume in each use, providing reproducible results.
These tests are critical to ensuring that droppers used in pharmaceutical and laboratory settings dispense a consistent volume of liquid with each use.
Quality Control Processes for Droppers
Droppers must undergo various quality control processes to ensure reliable operation. One of the most critical steps in these processes is cleaning the dropper. An unclean dropper can affect the liquid flow and the drop weight, so the dropper should be carefully cleaned before testing. It is also essential that the water used in the test is at 20 ± 1 °C since water temperature affects the density and, consequently, the weight of the drops.
Applications of Droppers
Droppers are used in various fields where precise liquid measurement is crucial. The main applications include:
- Pharmaceutical Applications: Droppers play a critical role in the precise dosing of medications, such as eye drops and liquid medications taken orally.
- Laboratory Analyses: Droppers are widely used in techniques requiring precise liquid measurement, such as titration.
- Biomedical Research: Droppers are also used in biomedical research for tasks like cell culture and biological sample preparation, where small quantities of liquid transfer are required.
The reliable operation of droppers is essential in these fields to ensure the accuracy of measurement results.
References
- European Pharmacopoeia. (2021). Droppers: Standard Specifications and Testing Protocols.
- Fuchs, M., & Müller, P. (2019). Pharmaceutical Dosage Forms: A Handbook for Laboratory Practices. Springer Press.
- World Health Organization. (2020). Quality Control Standards for Laboratory Equipment and Tools.
 EUP standards, the following tests are conducted to determine the compliance of a dropper:
EUP standards, the following tests are conducted to determine the compliance of a dropper: